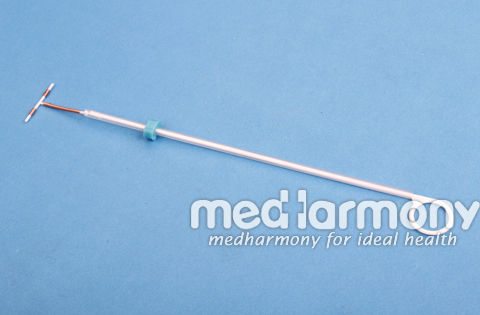
IUD(Intrauterine Device)
Product Name: IUD (Intrauterine Device) Cu 380A
Product Size: 26mm, 28mm, 30mm, 32mm
Product Description: T Care 380A wish safe load
Each unit wound with approximately 176mg of copper wire in addition a single copper sleeve is swaged on each of the two transverse arms, each sleeve contains approximately 66.5mg of copper. The total surface area of copper on the device is 380±23mm2.
Sterilization by EO.
Warning: STERILE unless the package is opened or damaged; insertion instrument should not be reused and should be destroyed after use.
Administration: To be inserted in the uterus only by or under the supervision of a physician. See detailed instructions for use. It is recommended that the unit be replaced by 5years from the date of insertion.
- Previous:Disposable Medical Razor
Next:Apron PE